What Are Critical Limits of Beef
Adopted August 14, 1997
NATIONAL Advisory COMMITTEE ON MICROBIOLOGICAL CRITERIA FOR FOODS
The National Advisory Committee on Microbiological Criteria for Foods (NACMCF) is an advisory committee chartered under the U.S. Department of Agriculture (USDA) and comprised of participants from the USDA (Food Safety and Inspection Service), Department of Health and Human Services (U.South. Food and Drug Administration and the Centers for Disease Command and Prevention) the Department of Commerce (National Marine Fisheries Service), the Section of Defence force (Office of the Army Surgeon General), academia, industry and country employees. NACMCF provides guidance and recommendations to the Secretary of Agriculture and the Secretarial assistant of Wellness and Human Services regarding the microbiological safety of foods.
Table of Contents
EXECUTIVE SUMMARY
DEFINITIONS
HACCP PRINCIPLES
GUIDELINES FOR APPLICATION OF HACCP PRINCIPLES
- Introduction
- Prerequisite Programs
- Educational activity and Preparation
- Developing a HACCP Program
- Assemble the HACCP squad
- Describe the food and its distribution
- Describe the intended utilize and consumers of the food
- Develop a menses diagram which describes the procedure
- Verify the flow diagram
- Acquit a hazard analysis (Principle 1)
- Decide critical control points (CCPs) (Principle 2)
- Establish disquisitional limits (Principle 3)
- Establish monitoring procedures (Principle four)
- Establish cosmetic actions (Principle v)
- Establish verification procedures (Principle vi)
- Found tape-keeping and documentation procedures (Principle 7)
IMPLEMENTATION AND MAINTENANCE OF THE HACCP Programme
APPENDIX A - Examples of common prerequisite programs
APPENDIX B - Case of a menstruum diagram for the product of frozen cooked beef patties.
APPENDIX C - Examples of questions to be considered when conducting a run a risk analysis
APPENDIX D - Examples of how the stages of hazard analysis are used to identify and evaluate hazards
APPENDIX Due east - Example I of a CCP decision tree
APPENDIX F - Example II of a CCP determination tree
APPENDIX G - Examples of verification activities
APPENDIX H - Examples of HACCP records
EXECUTIVE SUMMARY
The National Advisory Commission on Microbiological Criteria for Foods (Committee) reconvened a Hazard Analysis and Disquisitional Control Point (HACCP) Working Grouping in 1995. The primary goal was to review the Commission's November 1992 HACCP document, comparing it to electric current HACCP guidance prepared by the Codex Committee on Food Hygiene. Based upon its review, the Commission made the HACCP principles more concise; revised and added definitions; included sections on prerequisite programs, educational activity and training, and implementation and maintenance of the HACCP plan; revised and provided a more detailed caption of the application of HACCP principles; and provided an additional determination tree for identifying critical command points (CCPs).
The Committee once more endorses HACCP as an effective and rational means of assuring food safety from harvest to consumption. Preventing problems from occurring is the paramount goal underlying any HACCP system. Seven bones principles are employed in the development of HACCP plans that see the stated goal. These principles include adventure analysis, CCP identification, establishing critical limits, monitoring procedures, corrective actions, verification procedures, and tape-keeping and documentation. Nether such systems, if a deviation occurs indicating that control has been lost, the deviation is detected and appropriate steps are taken to reestablish control in a timely style to assure that potentially hazardous products do not reach the consumer.
In the awarding of HACCP, the use of microbiological testing is seldom an effective means of monitoring CCPs because of the fourth dimension required to obtain results. In most instances, monitoring of CCPs can best be accomplished through the use of concrete and chemical tests, and through visual observations. Microbiological criteria practise, however, play a role in verifying that the overall HACCP system is working.
The Commission believes that the HACCP principles should be standardized to provide uniformity in preparation and applying the HACCP system past industry and government. In accordance with the National Academy of Sciences recommendation, the HACCP system must exist developed by each food establishment and tailored to its individual product, processing and distribution conditions.
In keeping with the Committee's charge to provide recommendations to its sponsoring agencies regarding microbiological food safe issues, this certificate focuses on this surface area. The Commission recognizes that in order to clinch nutrient safe, properly designed HACCP systems must also consider chemical and concrete hazards in addition to other biological hazards.
For a successful HACCP program to be properly implemented, management must be committed to a HACCP arroyo. A commitment by management volition point an awareness of the benefits and costs of HACCP and include education and training of employees. Benefits, in addition to enhanced assurance of food safety, are ameliorate use of resources and timely response to problems.
The Committee designed this document to guide the food industry and advise its sponsoring agencies in the implementation of HACCP systems.
DEFINITIONS
CCP Decision Tree: A sequence of questions to assist in determining whether a control signal is a CCP.
Control: (a) To manage the conditions of an operation to maintain compliance with established criteria. (b) The state where correct procedures are beingness followed and criteria are being met.
Command Measure out: Any activeness or activeness that can exist used to forestall, eliminate or reduce a significant take a chance.
Control Point: Any step at which biological, chemic, or physical factors tin can be controlled.
Corrective Action: Procedures followed when a divergence occurs.
Criterion: A requirement on which a judgement or decision can be based.
Critical Control Point: A step at which control tin can be practical and is essential to foreclose or eliminate a food safety run a risk or reduce it to an acceptable level.
Disquisitional Limit: A maximum and/or minimum value to which a biological, chemical or physical parameter must be controlled at a CCP to prevent, eliminate or reduce to an acceptable level the occurrence of a nutrient prophylactic take a chance.
Difference: Failure to meet a critical limit.
HACCP: A systematic approach to the identification, evaluation, and control of food safety hazards.
HACCP Plan: The written document which is based upon the principles of HACCP and which delineates the procedures to be followed.
HACCP System: The effect of the implementation of the HACCP Plan.
HACCP Team: The group of people who are responsible for developing, implementing and maintaining the HACCP system.
Hazard: A biological, chemical, or concrete agent that is reasonably likely to cause illness or injury in the absence of its control.
Take a chance Analysis: The process of collecting and evaluating information on hazards associated with the food nether consideration to decide which are meaning and must be addressed in the HACCP plan.
Monitor: To behave a planned sequence of observations or measurements to assess whether a CCP is nether control and to produce an accurate record for future apply in verification.
Prerequisite Programs: Procedures, including Good Manufacturing Practices, that address operational conditions providing the foundation for the HACCP organisation.
Severity: The seriousness of the effect(due south) of a hazard.
Pace: A point, procedure, functioning or stage in the nutrient arrangement from primary production to concluding consumption.
Validation: That element of verification focused on collecting and evaluating scientific and technical information to make up one's mind if the HACCP program, when properly implemented, will effectively command the hazards.Verification: Those activities, other than monitoring, that determine the validity of the HACCP plan and that the system is operating according to the plan.
HACCP PRINCIPLES
HACCP is a systematic approach to the identification, evaluation, and control of food safety hazards based on the following seven principles:
Principle ane: Conduct a hazard analysis.
Principle 2: Determine the disquisitional control points (CCPs).
Principle 3: Institute disquisitional limits.
Principle four: Found monitoring procedures.
Principle 5: Establish cosmetic actions.
Principle 6: Constitute verification procedures.
Principle vii: Establish record-keeping and documentation procedures.
GUIDELINES FOR APPLICATION OF HACCP PRINCIPLES
Introduction
HACCP is a management system in which food safety is addressed through the analysis and control of biological, chemical, and concrete hazards from raw material production, procurement and handling, to manufacturing, distribution and consumption of the finished product. For successful implementation of a HACCP plan, management must be strongly committed to the HACCP concept. A firm commitment to HACCP by top management provides company employees with a sense of the importance of producing safe food.
HACCP is designed for use in all segments of the food manufacture from growing, harvesting, processing, manufacturing, distributing, and merchandising to preparing food for consumption. Prerequisite programs such as electric current Skilful Manufacturing Practices (cGMPs) are an essential foundation for the evolution and implementation of successful HACCP plans. Food prophylactic systems based on the HACCP principles have been successfully applied in nutrient processing plants, retail food stores, and food service operations. The seven principles of HACCP accept been universally accepted by government agencies, trade associations and the food industry around the earth.
The following guidelines will facilitate the development and implementation of effective HACCP plans. While the specific application of HACCP to manufacturing facilities is emphasized here, these guidelines should exist practical as advisable to each segment of the nutrient industry under consideration.
Prerequisite Programs
The product of safe food products requires that the HACCP system be built upon a solid foundation of prerequisite programs. Examples of mutual prerequisite programs are listed in Appendix A. Each segment of the nutrient industry must provide the atmospheric condition necessary to protect food while it is under their command. This has traditionally been achieved through the application of cGMPs. These weather condition and practices are now considered to be prerequisite to the development and implementation of effective HACCP plans. Prerequisite programs provide the basic ecology and operating atmospheric condition that are necessary for the product of condom, wholesome food. Many of the weather condition and practices are specified in federal, country and local regulations and guidelines (e.g., cGMPs and Food Code). The Codex Alimentarius General Principles of Food Hygiene describe the basic conditions and practices expected for foods intended for international trade. In improver to the requirements specified in regulations, industry often adopts policies and procedures that are specific to their operations. Many of these are proprietary. While prerequisite programs may touch on upon the safety of a nutrient, they also are concerned with ensuring that foods are wholesome and suitable for consumption (Appendix A). HACCP plans are narrower in telescopic, existence limited to ensuring nutrient is safe to consume.
The existence and effectiveness of prerequisite programs should be assessed during the design and implementation of each HACCP plan. All prerequisite programs should exist documented and regularly audited. Prerequisite programs are established and managed separately from the HACCP plan. Certain aspects, however, of a prerequisite programme may be incorporated into a HACCP plan. For example, many establishments have preventive maintenance procedures for processing equipment to avert unexpected equipment failure and loss of production. During the development of a HACCP plan, the HACCP team may decide that the routine maintenance and calibration of an oven should exist included in the programme equally an activeness of verification. This would further ensure that all the nutrient in the oven is cooked to the minimum internal temperature that is necessary for food prophylactic.
Education and Preparation
The success of a HACCP system depends on educating and training direction and employees in the importance of their function in producing safety foods. This should also include information the control of foodborne hazards related to all stages of the food chain. It is of import to recognize that employees must first empathize what HACCP is and and so learn the skills necessary to make information technology role properly. Specific training activities should include working instructions and procedures that outline the tasks of employees monitoring each CCP.
Management must provide adequate time for thorough education and training. Personnel must exist given the materials and equipment necessary to perform these tasks. Effective training is an important prerequisite to successful implementation of a HACCP plan.
Developing a HACCP Plan
The format of HACCP plans will vary. In many cases the plans will exist production and process specific. However, some plans may use a unit operations approach. Generic HACCP plans tin serve as useful guides in the development of procedure and production HACCP plans; however, it is essential that the unique weather inside each facility be considered during the evolution of all components of the HACCP program.
In the development of a HACCP plan, five preliminary tasks need to exist accomplished before the application of the HACCP principles to a specific production and procedure. The five preliminary tasks are given in Figure 1.
Effigy 1. Preliminary Tasks in the Evolution of the HACCP Plan
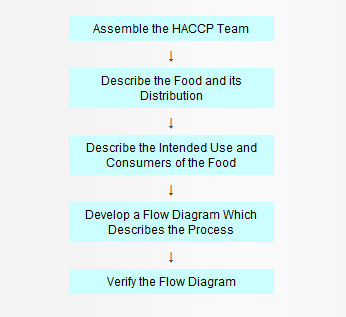
Get together the HACCP Team
The first job in developing a HACCP programme is to assemble a HACCP squad consisting of individuals who have specific knowledge and expertise appropriate to the product and procedure. Information technology is the team'southward responsibleness to develop the HACCP plan. The team should be multi disciplinary and include individuals from areas such equally engineering, production, sanitation, quality balls, and food microbiology. The team should too include local personnel who are involved in the operation every bit they are more than familiar with the variability and limitations of the operation. In improver, this fosters a sense of buying among those who must implement the program. The HACCP team may need help from outside experts who are knowledgeable in the potential biological, chemical and/or physical hazards associated with the product and the process. However, a plan which is developed totally by exterior sources may be erroneous, incomplete, and lacking in support at the local level.
Due to the technical nature of the information required for risk assay, it is recommended that experts who are knowledgeable in the food process should either participate in or verify the completeness of the run a risk analysis and the HACCP plan. Such individuals should accept the knowledge and experience to correctly: (a) conduct a hazard analysis; (b) identify potential hazards; (c) place hazards which must exist controlled; (d) recommend controls, disquisitional limits, and procedures for monitoring and verification; (e) recommend advisable cosmetic actions when a deviation occurs; (f) recommend inquiry related to the HACCP program if important information is not known; and (g) validate the HACCP plan.
Describe the nutrient and its distribution
The HACCP team showtime describes the nutrient. This consists of a general description of the food, ingredients, and processing methods. The method of distribution should be described along with information on whether the food is to be distributed frozen, refrigerated, or at ambient temperature.
Describe the intended apply and consumers of the food
Describe the normal expected use of the nutrient. The intended consumers may be the full general public or a particular segment of the population (e.g., infants, immunocompromised individuals, the elderly, etc.).
Develop a flow diagram which describes the process
The purpose of a period diagram is to provide a clear, simple outline of the steps involved in the process. The scope of the flow diagram must cover all the steps in the procedure which are directly under the control of the establishment. In improver, the menses diagram tin can include steps in the food chain which are earlier and after the processing that occurs in the establishment. The period diagram demand not be equally complex as engineering drawings. A block blazon flow diagram is sufficiently descriptive (see Appendix B). Also, a simple schematic of the facility is often useful in agreement and evaluating product and process flow.
Verify the flow diagram
The HACCP team should perform an on-site review of the functioning to verify the accuracy and completeness of the flow diagram. Modifications should be made to the flow diagram every bit necessary and documented.
Afterward these five preliminary tasks take been completed, the seven principles of HACCP are applied.
Bear a take a chance analysis (Principle 1)
After addressing the preliminary tasks discussed above, the HACCP squad conducts a chance analysis and identifies appropriate control measures. The purpose of the take chances assay is to develop a list of hazards which are of such significance that they are reasonably probable to cause injury or illness if not effectively controlled. Hazards that are not reasonably probable to occur would non require further consideration within a HACCP plan. It is of import to consider in the hazard analysis the ingredients and raw materials, each step in the process, product storage and distribution, and final preparation and use by the consumer. When conducting a chance analysis, safety concerns must be differentiated from quality concerns. A hazard is divers as a biological, chemical or physical agent that is reasonably likely to crusade disease or injury in the absence of its command. Thus, the discussion hazard as used in this document is express to safe.
A thorough risk analysis is the key to preparing an constructive HACCP program. If the hazard analysis is non done correctly and the hazards warranting control within the HACCP system are non identified, the plan will not be effective regardless of how well it is followed.
The hazard analysis and identification of associated command measures accomplish three objectives: Those hazards and associated control measures are identified. The analysis may place needed modifications to a process or production so that product safety is further assured or improved. The analysis provides a basis for determining CCPs in Principle two.
The process of conducting a chance analysis involves ii stages. The beginning, risk identification, can be regarded equally a brain storming session. During this stage, the HACCP team reviews the ingredients used in the product, the activities conducted at each step in the process and the equipment used, the final product and its method of storage and distribution, and the intended apply and consumers of the production. Based on this review, the team develops a list of potential biological, chemical or physical hazards which may be introduced, increased, or controlled at each footstep in the product process. Appendix C lists examples of questions that may be helpful to consider when identifying potential hazards. Run a risk identification focuses on developing a listing of potential hazards associated with each process pace under direct control of the food performance. A noesis of whatever adverse health-related events historically associated with the product will exist of value in this practice.
After the list of potential hazards is assembled, stage two, the hazard evaluation, is conducted. In stage ii of the hazard analysis, the HACCP team decides which potential hazards must be addressed in the HACCP program. During this stage, each potential hazard is evaluated based on the severity of the potential hazard and its likely occurrence. Severity is the seriousness of the consequences of exposure to the hazard. Considerations of severity (eastward.g., impact of sequelae, and magnitude and duration of illness or injury) can be helpful in understanding the public health impact of the hazard. Consideration of the likely occurrence is usually based upon a combination of experience, epidemiological information, and information in the technical literature. When conducting the risk evaluation, it is helpful to consider the likelihood of exposure and severity of the potential consequences if the gamble is non properly controlled. In add-on, consideration should be given to the effects of short term likewise every bit long term exposure to the potential hazard. Such considerations practise not include common dietary choices which lie exterior of HACCP. During the evaluation of each potential take a chance, the food, its method of preparation, transportation, storage and persons likely to consume the product should be considered to determine how each of these factors may influence the likely occurrence and severity of the risk being controlled. The squad must consider the influence of likely procedures for food training and storage and whether the intended consumers are susceptible to a potential run a risk. However, there may be differences of opinion, fifty-fifty among experts, as to the likely occurrence and severity of a gamble. The HACCP team may have to rely upon the stance of experts who aid in the development of the HACCP plan.
Hazards identified in ane operation or facility may non be significant in some other operation producing the aforementioned or a like production. For example, due to differences in equipment and/or an constructive maintenance plan, the probability of metal contamination may exist significant in one facility simply not in some other. A summary of the HACCP team deliberations and the rationale developed during the take a chance analysis should exist kept for hereafter reference. This information will be useful during future reviews and updates of the run a risk analysis and the HACCP plan.
Appendix D gives three examples of using a logic sequence in conducting a run a risk assay. While these examples relate to biological hazards, chemical and physical hazards are every bit important to consider. Appendix D is for illustration purposes to further explicate the stages of hazard analysis for identifying hazards. Risk identification and evaluation as outlined in Appendix D may eventually be assisted by biological risk assessments equally they get available. While the procedure and output of a risk assessment (NACMCF, 1997)(1) is significantly dissimilar from a take chances analysis, the identification of hazards of business organisation and the hazard evaluation may be facilitated past information from hazard assessments. Thus, as run a risk assessments addressing specific hazards or control factors become available, the HACCP squad should take these into consideration.
Upon completion of the take chances analysis, the hazards associated with each footstep in the production of the nutrient should be listed along with whatsoever measure out(s) that are used to control the run a risk(southward). The term control mensurate is used because not all hazards can be prevented, but virtually all tin be controlled. More than one control measure may be required for a specific hazard. On the other manus, more than 1 hazard may be addressed by a specific control measure (e.1000. pasteurization of milk).
For instance, if a HACCP team were to carry a risk analysis for the production of frozen cooked beefiness patties (Appendices B and D), enteric pathogens (due east.k., Salmonella and verotoxin-producing Escherichia coli) in the raw meat would be identified as hazards. Cooking is a control measure which can exist used to eliminate these hazards. The post-obit is an excerpt from a hazard assay summary table for this product.
| Step | Potential Hazard(s) | Justification | Hazard to exist addressed in plan? | Command |
|---|---|---|---|---|
| 5. Cooking | Enteric pathogens: | enteric pathogens take been associated with outbreaks of foodborne illness from undercooked basis beef | Y | Cooking |
The hazard analysis summary could exist presented in several different ways. One format is a table such every bit the i given above. Another could be a narrative summary of the HACCP squad'south hazard analysis considerations and a summary table list simply the hazards and associated control measures.
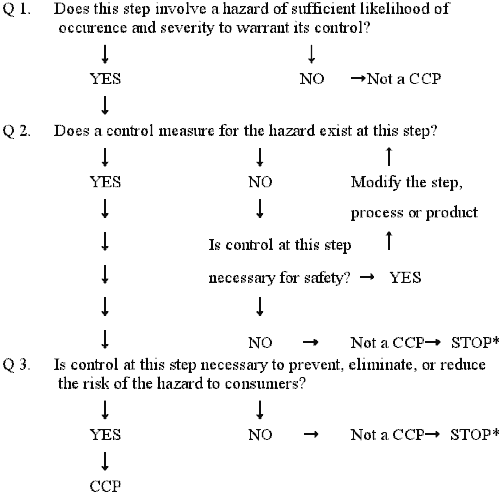
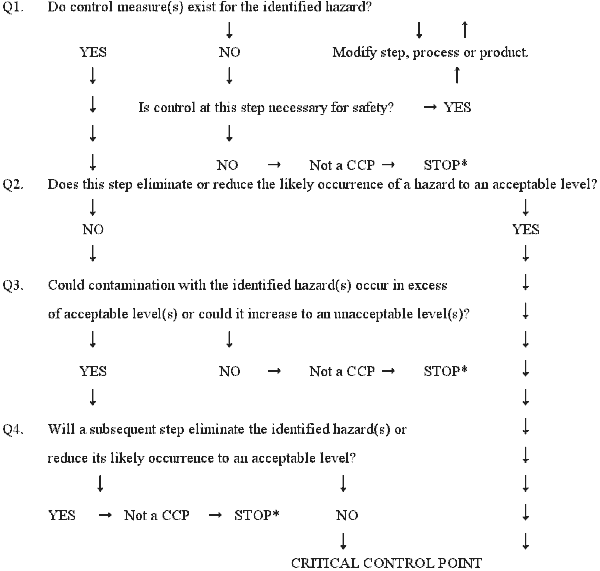
Make up one's mind critical control points (CCPs) (Principle ii)
A critical command point is defined as a step at which command can exist applied and is essential to prevent or eliminate a food safety hazard or reduce it to an acceptable level. The potential hazards that are reasonably probable to cause disease or injury in the absence of their control must be addressed in determining CCPs.
Complete and accurate identification of CCPs is central to decision-making food rubber hazards. The information developed during the gamble assay is essential for the HACCP team in identifying which steps in the process are CCPs. One strategy to facilitate the identification of each CCP is the apply of a CCP decision tree (Examples of conclusion trees are given in Appendices Eastward and F). Although awarding of the CCP decision tree can exist useful in determining if a particular step is a CCP for a previously identified take chances, it is merely a tool and not a mandatory element of HACCP. A CCP decision tree is not a substitute for expert knowledge.
Critical control points are located at any step where hazards can be either prevented, eliminated, or reduced to acceptable levels. Examples of CCPs may include: thermal processing, chilling, testing ingredients for chemic residues, production formulation control, and testing product for metal contaminants. CCPs must be carefully adult and documented. In addition, they must be used merely for purposes of product safe. For example, a specified heat process, at a given time and temperature designed to destroy a specific microbiological pathogen, could exist a CCP. Besides, refrigeration of a precooked food to prevent hazardous microorganisms from multiplying, or the adjustment of a food to a pH necessary to prevent toxin formation could as well be CCPs. Unlike facilities preparing like nutrient items can differ in the hazards identified and the steps which are CCPs. This can be due to differences in each facility's layout, equipment, selection of ingredients, processes employed, etc.
Establish disquisitional limits (Principle 3)
A disquisitional limit is a maximum and/or minimum value to which a biological, chemic or physical parameter must be controlled at a CCP to prevent, eliminate or reduce to an acceptable level the occurrence of a food safety gamble. A critical limit is used to distinguish betwixt safe and unsafe operating conditions at a CCP. Critical limits should not be dislocated with operational limits which are established for reasons other than food safety.
Each CCP will take one or more control measures to assure that the identified hazards are prevented, eliminated or reduced to adequate levels. Each control mensurate has i or more than associated disquisitional limits. Critical limits may be based upon factors such as: temperature, time, concrete dimensions, humidity, moisture level, h2o activity (awestward), pH, titratable acidity, common salt concentration, available chlorine, viscosity, preservatives, or sensory information such every bit aroma and visual appearance. Critical limits must be scientifically based. For each CCP, there is at least one criterion for food safety that is to be met. An example of a criterion is a specific lethality of a cooking process such equally a 5D reduction in Salmonella. The disquisitional limits and criteria for food prophylactic may be derived from sources such as regulatory standards and guidelines, literature surveys, experimental results, and experts.
An instance is the cooking of beef patties (Appendix B). The process should be designed to ensure the production of a prophylactic production. The adventure analysis for cooked meat patties identified enteric pathogens (e.thousand., verotoxigenic Eastward. coli such as E. coli O157:H7, and salmonellae) as meaning biological hazards. Furthermore, cooking is the pace in the process at which control tin can exist applied to reduce the enteric pathogens to an acceptable level. To ensure that an acceptable level is consistently achieved, accurate information is needed on the likely number of the pathogens in the raw patties, their estrus resistance, the factors that influence the heating of the patties, and the area of the patty which heats the slowest. Collectively, this information forms the scientific ground for the critical limits that are established. Some of the factors that may affect the thermal destruction of enteric pathogens are listed in the following tabular array. In this example, the HACCP squad ended that a thermal process equivalent to 155° F for xvi seconds would be necessary to assure the condom of this product. To ensure that this time and temperature are attained, the HACCP squad for i facility determined that it would be necessary to plant critical limits for the oven temperature and humidity, belt speed (time in oven), patty thickness and composition (east.g., all beef, beef and other ingredients). Control of these factors enables the facility to produce a wide variety of cooked patties, all of which volition be candy to a minimum internal temperature of 155° F for 16 seconds. In another facility, the HACCP team may conclude that the best arroyo is to apply the internal patty temperature of 155° F and hold for sixteen seconds as critical limits. In this second facility the internal temperature and hold fourth dimension of the patties are monitored at a frequency to ensure that the critical limits are constantly met as they exit the oven. The example given below applies to the first facility.
| Procedure Step | CCP | Critical Limits |
|---|---|---|
| v. Cooking | Yep | Oven temperature:___° F |
Plant monitoring procedures (Principle 4)
Monitoring is a planned sequence of observations or measurements to assess whether a CCP is under control and to produce an authentic record for future utilize in verification. Monitoring serves 3 master purposes. Beginning, monitoring is essential to food rubber management in that it facilitates tracking of the functioning. If monitoring indicates that in that location is a trend towards loss of control, and so action tin exist taken to bring the procedure back into control before a difference from a critical limit occurs. Second, monitoring is used to determine when there is loss of command and a divergence occurs at a CCP, i.e., exceeding or not meeting a critical limit. When a deviation occurs, an appropriate corrective action must be taken. Third, it provides written documentation for apply in verification.
An unsafe food may result if a process is not properly controlled and a deviation occurs. Considering of the potentially serious consequences of a critical limit deviation, monitoring procedures must exist effective. Ideally, monitoring should be continuous, which is possible with many types of concrete and chemical methods. For example, the temperature and time for the scheduled thermal procedure of low-acrid canned foods is recorded continuously on temperature recording charts. If the temperature falls beneath the scheduled temperature or the time is insufficient, as recorded on the chart, the product from the retort is retained and the disposition determined as in Principle 5. Also, pH measurement may be performed continually in fluids or by testing each batch before processing. There are many ways to monitor critical limits on a continuous or batch basis and tape the data on charts. Continuous monitoring is always preferred when feasible. Monitoring equipment must be carefully calibrated for accuracy.
Assignment of the responsibility for monitoring is an important consideration for each CCP. Specific assignments will depend on the number of CCPs and command measures and the complexity of monitoring. Personnel who monitor CCPs are often associated with production (e.g., line supervisors, selected line workers and maintenance personnel) and, as required, quality control personnel. Those individuals must be trained in the monitoring technique for which they are responsible, fully understand the purpose and importance of monitoring, be unbiased in monitoring and reporting, and accurately written report the results of monitoring. In addition, employees should be trained in procedures to follow when in that location is a trend towards loss of control and so that adjustments can exist fabricated in a timely manner to clinch that the process remains under control. The person responsible for monitoring must too immediately report a process or product that does not run across critical limits.
All records and documents associated with CCP monitoring should be dated and signed or initialed by the person doing the monitoring.
When it is not possible to monitor a CCP on a continuous basis, information technology is necessary to establish a monitoring frequency and procedure that will be reliable enough to indicate that the CCP is nether control. Statistically designed information collection or sampling systems lend themselves to this purpose.
Most monitoring procedures need to be rapid because they relate to on-line, "real-time" processes and there volition not be time for lengthy belittling testing. Examples of monitoring activities include: visual observations and measurement of temperature, time, pH, and moisture level.
Microbiological tests are seldom effective for monitoring due to their fourth dimension-consuming nature and problems with assuring detection of contaminants. Physical and chemical measurements are oft preferred because they are rapid and usually more effective for assuring control of microbiological hazards. For example, the safety of pasteurized milk is based upon measurements of time and temperature of heating rather than testing the heated milk to assure the absenteeism of surviving pathogens.
With certain foods, processes, ingredients, or imports, there may exist no alternative to microbiological testing. However, it is important to recognize that a sampling protocol that is acceptable to reliably detect low levels of pathogens is seldom possible because of the large number of samples needed. This sampling limitation could result in a false sense of security by those who use an inadequate sampling protocol. In add-on, in that location are technical limitations in many laboratory procedures for detecting and quantitating pathogens and/or their toxins.
Institute cosmetic actions (Principle five)
The HACCP system for food prophylactic direction is designed to identify health hazards and to establish strategies to foreclose, eliminate, or reduce their occurrence. Still, ideal circumstances practice non e'er prevail and deviations from established processes may occur. An of import purpose of cosmetic actions is to prevent foods which may be hazardous from reaching consumers. Where there is a deviation from established critical limits, corrective deportment are necessary. Therefore, corrective actions should include the following elements: (a) decide and right the cause of not-compliance; (b) determine the disposition of non-compliant product and (c) record the corrective actions that take been taken. Specific corrective actions should be developed in advance for each CCP and included in the HACCP plan. As a minimum, the HACCP plan should specify what is done when a departure occurs, who is responsible for implementing the corrective actions, and that a record volition be developed and maintained of the actions taken. Individuals who have a thorough agreement of the process, product and HACCP program should be assigned the responsibility for oversight of corrective deportment. Every bit appropriate, experts may be consulted to review the information available and to aid in determining disposition of non-compliant product.
Establish verification procedures (Principle 6)
Verification is defined every bit those activities, other than monitoring, that make up one's mind the validity of the HACCP programme and that the system is operating according to the program. The NAS (1985) (2) pointed out that the major infusion of scientific discipline in a HACCP organisation centers on proper identification of the hazards, critical command points, critical limits, and instituting proper verification procedures. These processes should take identify during the evolution and implementation of the HACCP plans and maintenance of the HACCP organisation. An example of a verification schedule is given in Figure 2.
Ane aspect of verification is evaluating whether the facility'southward HACCP system is functioning according to the HACCP plan. An constructive HACCP system requires niggling cease-product testing, since sufficient validated safeguards are built in early in the process. Therefore, rather than relying on end-product testing, firms should rely on frequent reviews of their HACCP program, verification that the HACCP plan is existence correctly followed, and review of CCP monitoring and corrective activity records.
Another important aspect of verification is the initial validation of the HACCP plan to determine that the plan is scientifically and technically audio, that all hazards accept been identified and that if the HACCP plan is properly implemented these hazards volition be effectively controlled. Data needed to validate the HACCP plan ofttimes include (1) expert advice and scientific studies and (2) in-constitute observations, measurements, and evaluations. For example, validation of the cooking process for beef patties should include the scientific justification of the heating times and temperatures needed to obtain an appropriate destruction of pathogenic microorganisms (i.eastward., enteric pathogens) and studies to ostend that the conditions of cooking will deliver the required time and temperature to each beef patty.
Subsequent validations are performed and documented by a HACCP squad or an independent expert every bit needed. For case, validations are conducted when there is an unexplained system failure; a significant production, procedure or packaging modify occurs; or new hazards are recognized.
In add-on, a periodic comprehensive verification of the HACCP system should be conducted by an unbiased, contained say-so. Such authorities tin be internal or external to the food operation. This should include a technical evaluation of the hazard assay and each element of the HACCP plan as well equally on-site review of all menses diagrams and advisable records from operation of the programme. A comprehensive verification is independent of other verification procedures and must be performed to ensure that the HACCP plan is resulting in the control of the hazards. If the results of the comprehensive verification identifies deficiencies, the HACCP team modifies the HACCP plan equally necessary.
Verification activities are carried out by individuals within a visitor, third political party experts, and regulatory agencies. It is important that individuals doing verification have appropriate technical expertise to perform this office. The role of regulatory and industry in HACCP was farther described by the NACMCF (1994) (3).
Examples of verification activities are included as Appendix G.
Effigy 2. Case of a Company Established HACCP Verification Schedule
| Activity | Frequency | Responsibility | Reviewer |
|---|---|---|---|
| Verification Activities Scheduling | Yearly or Upon HACCP Arrangement Change | HACCP Coordinator | Plant Managing director |
| Initial Validation of HACCP Program | Prior to and During Initial Implementation of Plan | Independent Expert(s)(a) | HACCP Team |
| Subsequent validation of HACCP Program | When Critical Limits Changed, Significant Changes in Process, Equipment Inverse, After Arrangement Failure, etc. | Independent Expert(southward)(a) | HACCP Squad |
| Verification of CCP Monitoring as Described in the Plan (east.g., monitoring of patty cooking temperature) | According to HACCP Plan (east.one thousand., one time per shift) | Co-ordinate to HACCP Plan (east.yard., Line Supervisor) | According to HACCP Plan (e.g., Quality Control) |
| Review of Monitoring, Corrective Action Records to Prove Compliance with the Programme | Monthly | Quality Assurance | HACCP Team |
| Comprehensive HACCP System Verification | Yearly | Independent Good(s)(a) | Institute Director |
| (a) Done by others than the team writing and implementing the plan. May require boosted technical expertise also every bit laboratory and establish exam studies. | |||
Establish tape-keeping and documentation procedures (Principle vii)
Generally, the records maintained for the HACCP System should include the following:
-
A summary of the hazard analysis, including the rationale for determining hazards and control measures.
-
The HACCP Plan
List of the HACCP team and assigned responsibilities.
Description of the nutrient, its distribution, intended use, and consumer.
Verified flow diagram.
HACCP Plan Summary Table that includes information for:
Steps in the process that are CCPs
The adventure(s) of business organization.
Critical limits
Monitoring*
Corrective actions*
Verification procedures and schedule*
Record-keeping procedures*
* A cursory summary of position responsible for performing the activity and the procedures and frequency should be provided
The following is an example of a HACCP plan summary tabular array:
CCP
Hazards
Disquisitional limit(s)
Monitoring
Corrective Actions
Verification
Records
-
Back up documentation such as validation records.
-
Records that are generated during the performance of the plan.
Examples of HACCP records are given in Appendix H.
IMPLEMENTATION AND MAINTENANCE OF THE HACCP PLAN
The successful implementation of a HACCP program is facilitated past commitment from top management. The adjacent pace is to found a plan that describes the individuals responsible for developing, implementing and maintaining the HACCP system. Initially, the HACCP coordinator and team are selected and trained every bit necessary. The squad is and so responsible for developing the initial program and coordinating its implementation. Product teams tin be appointed to develop HACCP plans for specific products. An important aspect in developing these teams is to assure that they have appropriate training. The workers who will be responsible for monitoring need to exist adequately trained. Upon completion of the HACCP plan, operator procedures, forms and procedures for monitoring and cosmetic activity are developed. Often it is a skilful idea to develop a timeline for the activities involved in the initial implementation of the HACCP plan. Implementation of the HACCP system involves the continual awarding of the monitoring, record-keeping, corrective action procedures and other activities as described in the HACCP programme.
Maintaining an effective HACCP system depends largely on regularly scheduled verification activities. The HACCP program should be updated and revised as needed. An important aspect of maintaining the HACCP arrangement is to clinch that all individuals involved are properly trained and then they understand their role and tin can finer fulfill their responsibilities.
(1) National Advisory Committee on Microbiological Criteria for Foods. 1997. The principles of risk cess for illness caused by foodborne biological agents. Adopted April 4, 1997.
(2) An Evaluation of the Function of Microbiological Criteria for Foods and Food Ingredients. 1985. National Academy of Sciences, National University Printing, Washington, DC.
(3) National Advisory Committee on Microbiological Criteria for Foods. 1994. The role of regulatory agencies and industry in HACCP. Int. J. Food Microbiol. 21:187-195.
APPENDIX A
Examples of Common Prerequisite Programs
The production of safe food products requires that the HACCP system be built upon a solid foundation of prerequisite programs. Each segment of the nutrient manufacture must provide the conditions necessary to protect food while it is under their control. This has traditionally been accomplished through the application of cGMPs. These conditions and practices are at present considered to be prerequisite to the evolution and implementation of effective HACCP plans. Prerequisite programs provide the basic environmental and operating conditions that are necessary for the production of safety, wholesome nutrient. Mutual prerequisite programs may include, but are not limited to:
Facilities: The institution should be located, synthetic and maintained according to germ-free design principles. There should be linear product flow and traffic control to minimize cross-contagion from raw to cooked materials.
Supplier Command: Each facility should assure that its suppliers have in place effective GMP and food safety programs. These may be the subject area of continuing supplier guarantee and supplier HACCP system verification.
Specifications: There should be written specifications for all ingredients, products, and packaging materials.
Production Equipment: All equipment should be constructed and installed according to germ-free design principles. Preventive maintenance and scale schedules should be established and documented.
Cleaning and Sanitation: All procedures for cleaning and sanitation of the equipment and the facility should be written and followed. A chief sanitation schedule should be in place.
Personal Hygiene: All employees and other persons who enter the manufacturing establish should follow the requirements for personal hygiene.
Training: All employees should receive documented training in personal hygiene, GMP, cleaning and sanitation procedures, personal safe, and their part in the HACCP program.
Chemical Control: Documented procedures must exist in place to assure the segregation and proper use of non-food chemicals in the plant. These include cleaning chemicals, fumigants, and pesticides or baits used in or effectually the plant.
Receiving, Storage and Shipping: All raw materials and products should be stored under sanitary conditions and the proper environmental conditions such as temperature and humidity to assure their rubber and wholesomeness
Traceability and Remember: All raw materials and products should be lot-coded and a recall system in place so that rapid and consummate traces and recalls can exist done when a product retrieval is necessary.
Pest Control: Effective pest control programs should exist in place.
Other examples of prerequisite programs might include quality assurance procedures; standard operating procedures for sanitation, processes, product formulations and recipes; glass control; procedures for receiving, storage and aircraft; labeling; and employee food and ingredient handling practices.
APPENDIX B
Instance of a Menstruum Diagram for the Production of Frozen Cooked Beefiness Patties

APPENDIX C
Examples of Questions to be Considered When Conducting a Adventure Assay
The run a risk analysis consists of asking a series of questions which are appropriate to the process under consideration. The purpose of the questions is to assist in identifying potential hazards.
-
Ingredients
- Does the nutrient contain any sensitive ingredients that may present microbiological hazards (eastward.g., Salmonella, Staphylococcus aureus); chemic hazards (e.k., aflatoxin, antibiotic or pesticide residues); or concrete hazards (stones, glass, metal)?
- Are potable water, ice and steam used in formulating or in handling the food?
- What are the sources (eastward.g., geographical region, specific supplier)
-
Intrinsic Factors - Physical characteristics and composition (east.one thousand., pH, blazon of acidulants, fermentable saccharide, water action, preservatives) of the food during and after processing.
- What hazards may result if the food composition is non controlled?
- Does the food allow survival or multiplication of pathogens and/or toxin formation in the food during processing?
- Volition the food permit survival or multiplication of pathogens and/or toxin formation during subsequent steps in the food chain?
- Are there other similar products in the market place place? What has been the safe record for these products? What hazards have been associated with the products?
-
Procedures used for processing
- Does the procedure include a controllable processing footstep that destroys pathogens? If so, which pathogens? Consider both vegetative cells and spores.
- If the product is subject area to recontamination between processing (e.thousand., cooking, pasteurizing) and packaging which biological, chemical or physical hazards are likely to occur?
-
Microbial content of the nutrient
- What is the normal microbial content of the food?
- Does the microbial population change during the normal fourth dimension the food is stored prior to consumption?
- Does the subsequent modify in microbial population change the safety of the food?
- Do the answers to the above questions indicate a high likelihood of certain biological hazards?
-
Facility blueprint
- Does the layout of the facility provide an adequate separation of raw materials from set-to-eat (RTE) foods if this is of import to nutrient rubber? If non, what hazards should be considered every bit possible contaminants of the RTE products?
- Is positive air force per unit area maintained in product packaging areas? Is this essential for product safety?
- Is the traffic pattern for people and moving equipment a significant source of contamination?
-
Equipment pattern and utilise
- Will the equipment provide the time-temperature control that is necessary for safe food?
- Is the equipment properly sized for the book of food that will be processed?
- Tin can the equipment be sufficiently controlled and then that the variation in performance will be within the tolerances required to produce a safe nutrient?
- Is the equipment reliable or is information technology decumbent to frequent breakdowns?
- Is the equipment designed so that it can be easily cleaned and sanitized?
- Is there a chance for production contamination with hazardous substances; e.g., glass?
- What product safety devices are used to raise consumer safe?
- metal detectors
- magnets
- sifters
- filters
- screens
- thermometers
- bone removal devices
- dud detectors
- To what degree will normal equipment habiliment affect the likely occurrence of a physical adventure (e.one thousand., metal) in the product?
- Are allergen protocols needed in using equipment for different products?
-
Packaging
- Does the method of packaging affect the multiplication of microbial pathogens and/or the formation of toxins?
- Is the package clearly labeled "Keep Refrigerated" if this is required for safety?
- Does the package include instructions for the safe handling and preparation of the food by the end user?
- Is the packaging material resistant to harm thereby preventing the archway of microbial contamination?
- Are tamper-evident packaging features used?
- Is each package and instance legibly and accurately coded?
- Does each package contain the proper characterization?
- Are potential allergens in the ingredients included in the listing of ingredients on the characterization?
-
Sanitation
- Tin sanitation accept an impact upon the safety of the nutrient that is being processed?
- Tin can the facility and equipment be easily cleaned and sanitized to let the rubber handling of food?
- Is it possible to provide germ-free conditions consistently and fairly to assure rubber foods?
-
Employee health, hygiene and education
- Tin employee health or personal hygiene practices impact upon the safety of the food being processed?
- Practise the employees understand the process and the factors they must control to assure the preparation of prophylactic foods?
- Will the employees inform management of a problem which could affect upon safety of nutrient?
-
Conditions of storage between packaging and the stop user
- What is the likelihood that the food will be improperly stored at the incorrect temperature?
- Would an error in improper storage lead to a microbiologically unsafe nutrient?
-
Intended use
- Will the food be heated past the consumer?
- Will in that location likely be leftovers?
-
Intended consumer
- Is the food intended for the full general public?
- Is the nutrient intended for consumption by a population with increased susceptibility to disease (eastward.g., infants, the aged, the infirmed, immunocompromised individuals)?
- Is the nutrient to be used for institutional feeding or the home?
APPENDIX D
| Adventure Analysis Phase | Frozen cooked beef patties produced in a manufacturing constitute | Product containing eggs prepared for foodservice | Commercial frozen pre-cooked, boned chicken for further processing | |
|---|---|---|---|---|
| Stage aneDetermine potential Hazard hazards associated Identification with production | Enteric pathogens (i.e., E. coli O157:H7 and Salmonella) | Salmonella in finished production. | Staphylococcus aureus in finished product. | |
| Stage 2 Hazard Evaluation | Assess severity of wellness consequences if potential hazard is non properly controlled. | Epidemiological evidence indicates that these pathogens cause severe health effects including death among children and elderly. Undercooked beef patties have been linked to disease from these pathogens. | Salmonellosis is a food borne infection causing a moderate to severe illness that tin be caused by ingestion of only a few cells of Salmonella. | Certain strains of S. aureus produce an enterotoxin which can cause a moderate foodborne illness. |
| Determine likelihood of occurrence of potential adventure if not properly controlled. | E. coli O157:H7 is of very low probability and salmonellae is of moderate probability in raw meat. | Product is made with liquid eggs which have been associated with past outbreaks of salmonellosis. Recent problems with Salmonella serotype Enteritidis in eggs cause increased business organization. Probability of Salmonella in raw eggs cannot exist ruled out. If not effectively controlled, some consumers are likely to be exposed to Salmonella from this food. | Production may be contaminated with S. aureus due to human handling during boning of cooked chicken. Enterotoxin capable of causing affliction will only occur every bit South. aureus multiplies to most 1,000,000/k. Operating procedures during boning and subsequent freezing prevent growth of S. aureus, thus the potential for enterotoxin formation is very low. | |
| Using information to a higher place, determine if this potential hazard is to exist addressed in the HACCP plan. | The HACCP team decides that enteric pathogens are hazards for this product. Hazards must be addressed in the plan. | HACCP squad determines that if the potential gamble is not properly controlled, consumption of product is probable to result in an unacceptable health chance. Hazard must be addressed in the plan. | The HACCP team determines that the potential for enterotoxin formation is very depression. Even so, it is even so desirable to continue the initial number of S. aureus organisms low. Employee practices that minimize contamination, rapid carbon dioxide freezing and handling instructions have been adequate to control this potential adventure. Potential hazard does not need to exist addressed in programme. | |
* For illustrative purposes merely. The potential hazards identified may non exist the simply hazards associated with the products listed. The responses may be different for different establishments.
APPENDIX E
Example I of a CCP Decision Tree
Important considerations when using the conclusion tree:
-
The conclusion tree is used later on the chance analysis.
-
The determination tree then is used at the steps where a chance that must be addressed in the HACCP plan has been identified.
-
A subsequent step in the process may be more effective for decision-making a adventure and may be the preferred CCP.
-
More than 1 stride in a process may be involved in controlling a chance.
-
More than one run a risk may be controlled by a specific command measure out.
* Proceed to next step in the procedure.
APPENDIX F
Case 2 of a CCP Conclusion Tree
*Continue to adjacent stride in the described process
APPENDIX G
Examples of Verification Activities
-
Verification procedures may include:
- Establishment of appropriate verification schedules.
- Review of the HACCP programme for abyss.
- Confirmation of the accuracy of the flow diagram.
- Review of the HACCP system to decide if the facility is operating according to the HACCP plan.
- Review of CCP monitoring records.
- Review of records for deviations and corrective deportment.
- Validation of critical limits to confirm that they are adequate to control significant hazards.
- Validation of HACCP program, including on-site review.
- Review of modifications of the HACCP programme.
- Sampling and testing to verify CCPs.
-
Verification should exist conducted:
- Routinely, or on an unannounced footing, to assure CCPs are under control.
- When there are emerging concerns about the safety of the product.
- When foods take been implicated as a vehicle of foodborne disease.
- To ostend that changes have been implemented correctly after a HACCP plan has been modified.
- To appraise whether a HACCP plan should be modified due to a change in the process, equipment, ingredients, etc.
-
Verification reports may include information on the presence and adequacy of.
- The HACCP program and the person(s) responsible for administering and updating the HACCP plan.
- The records associated with CCP monitoring.
- Straight recording of monitoring information of the CCP while in operation.
- Certification that monitoring equipment is properly calibrated and in working lodge.
- Cosmetic actions for deviations.
- Sampling and testing methods used to verify that CCPs are nether control.
- Modifications to the HACCP plan.
- Preparation and knowledge of individuals responsible for monitoring CCPs.
- Validation activities.
APPENDIX H
Examples of HACCP Records
-
Ingredients for which disquisitional limits have been established.
- Supplier certification records documenting compliance of an ingredient with a critical limit.
- Processor audit records verifying supplier compliance.
- Storage records (e.thou., time, temperature) for when ingredient storage is a CCP.
-
Processing, storage and distribution records
- Information that establishes the efficacy of a CCP to maintain product safety.
- Data establishing the prophylactic shelf life of the production; if historic period of production can bear on safety.
- Records indicating compliance with critical limits when packaging materials, labeling or sealing specifications are necessary for food safe.
- Monitoring records.
- Verification records.
-
Deviation and corrective action records.
-
Employee grooming records that are pertinent to CCPs and the HACCP plan.
-
Documentation of the adequacy of the HACCP plan from a knowledgeable HACCP expert.
Source: https://www.fda.gov/food/hazard-analysis-critical-control-point-haccp/haccp-principles-application-guidelines